Vibrant Covid-19 Testing
Vibrant Covid-19 Testing
Vibrant America is creating tests to help detect the SARS-CoV-2 virus and better understand the spread of COVID-19
From identifying recent infections to whether you have recovered from an exposure, our tests are built to cover the disease span. With a comprehensive combination of PCR and advanced serology-based antibody testing, Vibrant can provide results in as short a time as 36 hours.
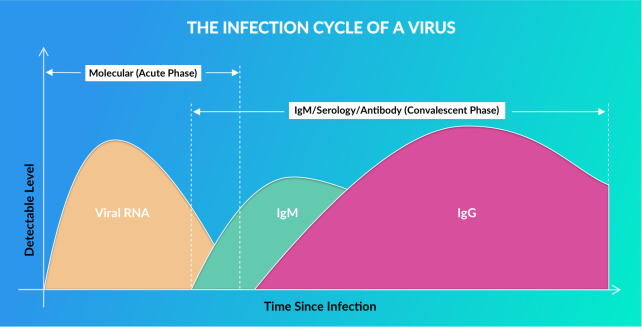
Viral Rna

Early Infection Phase:
Approximately 0-14 days after Infection
When the virus enters your body, it starts multiplying, and you may or may not have symptoms. As the virus multiples, levels of its molecular genetic material (RNA) rise, peak around 1 week, then fall off around 2 weeks.Molecular Tests:
Vibrant America is using a FDA EUA authorized real-time Polymerase Chain reaction (PCR) based test to detect viral SARS CoV-2 RNA present in nasal or nasopharyngeal specimen. Test Turnaround time is 24 hours from date of sample receipt.IGM Antibody
Later to End Infection Phase:
Approximately 1-8 weeks after Infection
As your body start to fight off infection, your immune system produces IgM antibodies. IgM antibodies generally appear several days after symptoms begin and can last 1-8 weeks.IgM Serology:
Vibrant America has developed and obtained FDA EUA for detection of IgM antibodies against 4 COVID-19 antigens using serum or dried blood spot. Turn-around time for testing is 36 hours from sample receipt.IGG Antibody
Post-Infection Phase:
Approximately weeks to months after Infection
As your body continues to fight off infection, it produces fewer IgM antibodies and more long-lasting IgG antibodies. IgG antibodies generally appear later than IgM antibodies.IgG Serology:
Vibrant America has developed and obtained FDA EUA for detection of IgG antibodies against 4 COVID-19 antigens using serum or dried blood spot. Turn-around time for testing is 36 hours from sample receipt.
Time periods listed for each phase of infection are general estimates and not indicative of a person’s immune reaction to SARS-CoV-2. These tests have not been FDA cleared or approved. The antibody tests have been authorized by FDA under EUA for use by authorized laboratories and have been authorized only for the detection of IgM and IgG antibodies against SARS CoV-2 and not for any other viruses or pathogens. The PCR tests is performed using TaqPath COVID-19 Combo Kit. The TaqPath COVID-19 Combo Kit is only for use under the Food and Drug Administration’s Emergency Use Authorization (EUA200010).
The tests are only authorized for the duration of the declaration that circumstances exist justifying the authorization of emergency use of in vitro diagnostic tests for detection and/or diagnosis of COVID-19 under Section 565(b)(1) of the Act, 21 U.S.C 360bbb-3(b)(1), unless the authorization is terminated or revoked sooner.


